Mechanical Freezing Systems
Any system that uses electrical power to produce chilled air. The chilled air is continuously passed over the food product, and in doing so, it removes heat. A large capital investment, a significant ongoing preventive maintenance cost, and a sizeable permanent commitment to plant space characterize mechanical freezing systems. On the other hand, the resulting refrigeration is produced discounted the consumable cost of cryogenic refrigeration. It is a widely used technology and is present in some form in virtually every food processing plant. Mechanical refrigeration systems sometimes tend to dehydrate or strip moisture from that product. For some products, this is not an issue. In others, it is a major quality or yield concern.
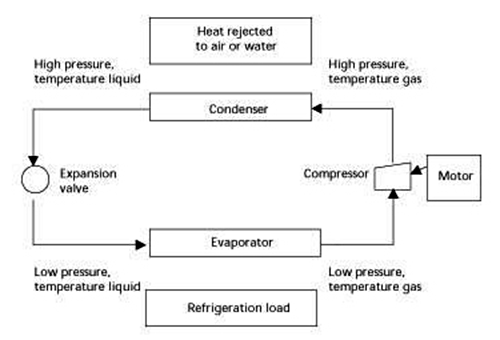
Mechanical Refrigeration
Refrigeration is the withdrawal of heat from a chamber (refrigeration load) to achieve temperatures lower than ambient temperatures. After, heat is withdrawn and transferred to a condenser and dissipated into air or water. The purpose of refrigeration in food processing is to preserve quality and delay spoilage; in volatile organic compounds recovery, it is to condense and capture harmful vapor emissions; and in the liquid natural gas industry, to facilitate natural gas storage and transportation.
90 percent of U.S. industrial refrigeration is provided by mechanical systems
More than 90 percent of U.S. industrial refrigeration is provided by mechanical systems using ammonia as the refrigerant. Mechanical refrigeration units are dedicated systems installed at individual industrial facilities and owned and operated by industrial companies. Refrigeration is achieved when the refrigerant, circulating in the system, withdraws heat energy from the chamber to be cooled (load). Heat energy (latent heat of evaporation) is absorbed as the liquid refrigerant changes to a gaseous state. Systems are composed of four basic elements connected with piping into a closed loop that re-circulates refrigerant. Compressors (generally) use motor-driven rotating impellers to generate gas pressure. Gaseous refrigerant enters the compressor at low pressure and temperature and exits at high pressure and temperature. Inside condenser coils, gaseous refrigerant condenses to a liquid state. The condenser dissipates heat energy to facilitate ambient air or water phase change. High-pressure refrigerant exits at lower temperatures. An expansion valve controls the flow of high-pressure liquid refrigerant to the evaporator. As the refrigerant passes through the expansion valve, it is further cooled by the Joule-Thompson effect, the scientific principle that the temperature of a stream is reduced when forced through a narrow nozzle and allowed to expand. Inside the evaporator, liquid refrigerant vaporizes into a gaseous state. Vaporization requires heat energy extracted from the industrial process load (food items to be cooled). The refrigerant is returned to the compressor to repeat the cycle.
High-Pressure Core Heat Exchanger
Heat exchangers are devices that transfer heat from a hot to a cold fluid. The barrier between the two fluids is a metal wall, such as that of a tube or pipe. In many engineering applications, increasing the temperature of one fluid while cooling another is desirable. This double action is economically accomplished by coils, evaporators, condensers, and coolers that may all be considered heat exchangers. Heat exchangers are designed with various flow arrangements. The concentric tube design uses one pipe placed inside another. Cold fluid flows through the inner tube, and warm fluid flows in the same direction through the annular space between the outer and the inner tube. Heat is transferred from the warm fluid through the wall of the inner tube (the so-called heating surface) to the cold fluid. Concentric tube heat exchangers can also be operated in counter-flow, where the two fluids flow in parallel but opposite directions. The shell and tube design utilizes a bundle of tubes through which one of the fluids flows. These tubes are enclosed in a shell with provisions for the other fluid to flow through the spaces between the tubes. In most designs of this type, the free fluid flows roughly perpendicular to the tubes containing the other fluid, in what is known as a cross-flow exchange. The plate-fin design uses metal sheets brazed together into internal channels to carry a warmer fluid stream, which is to be cooled. Fins, brazed to the outside surface of these channels, facilitate faster and more efficient heat transfer to the cold fluid stream on the outside of these channels.
Cryogenic Freezing Systems
Liquid nitrogen or liquid carbon dioxide is purchased and kept in a pressurized storage vessel. The cryogen is piped as a liquid into the freezer unit and applied directly to the product in various modes depending on the cryogen, freezer type, or food product. However, the cryogen is a consumable product and, except in very unusual circumstances, can only be used once. Cryogenic freezing systems are characterized by moderate capital investment, minimal preventive maintenance cost, and a smaller and more flexible commitment to plant space (cryogenic freezers can be installed or removed over a weekend). However, liquid nitrogen or liquid carbon dioxide pricing and availability vary based on the geographic location of the processor, and the cost of refrigeration purchased this way can be more than triple the cost of mechanically produced refrigeration. In contrast to mechanical freezing, cryogenic freezing tends to crust the outside of the product and prevent (insert pictures) excessive moisture loss. Depending on the product, this could be viewed as a quality advantage.
Major Types of Cryogenic Tunnels
Flat Belt Tunnel Freezer
The belt is flat the entire length of the freezer. It can be LN2 or CO2
Multi-pass Tunnel Freezer
The belt is flat the entire length of the freezer, but there are three (or more) stacked belts. At the end of the first tier, the product is discharged to the second tier, returned to the entrance end of the freezer, discharged again onto the third tier, and conveyed back to the exit end. It can be LN2 or CO2
Belt Tunnel Freezer Belts
The conveyor system is made up of three or more short inclined belts, which tumble the product from one flight to the next. This is excellent for small food products that tend to freeze together when touching. By keeping the individual pieces of food product in motion, large volumes of small food products such as diced meats, berries, or vegetables can be individually quickly frozen (IQF). It can be LN2 or CO2
Immersion Freezer
The food product is conveyed through a bath of liquid nitrogen. It is the fastest heat transfer rate available in cryogenic freezing but is characterized by lower cryogen efficiency rates (higher nitrogen usage) than those achieved with other types of tunnels. However, LN2 immersion freezers mated to spiral freezers (either cryogenic or mechanical) have proven to be highly effective in increasing productivity and yield (reduced dehydration costs) and preventing damage to the bottom of the food product from the spiral belt. LN2 only.
Spiral Freezer
The outward appearance of the spiral is that of a large square box. Inside, the belt spirals around a center drum, each layer a few inches higher. The product is normally fed into the bottom of the spiral and exits at the top of the box. Although heat transfer rates are slower than other cryogenic equipment, this unit can contain as much as 450 feet of belt, allowing for very high production rates. It can be LN2 or CO2
Cabinet (Batch) Freezer
It looks like a large cabinet. Food product is loaded onto trays, slid into a rack, and the rack is pushed into the freezer. The rack and product are removed from the cabinet after freezing is complete. The unit takes up very little floor space, but production is usually limited to a few hundred pounds per hour. Although this is not an inline freezer, its relatively low cost and versatility make this unit a favorite of start-up processors and new product development projects. It can be LN2 or CO2
Tumbler
A long, inclined, rotating tube. Using gravity and internal design to convey the product, this unit is used for small food products that tend to freeze together when touching. Like the freezer, it produces an IQF product. CO2 only
How LN2 Works in a Cryogenic Tunnel
Freezer Most straight belt nitrogen tunnels (the most common type) take advantage of the refrigeration value of converting liquid nitrogen to vapor (Latent Heat of vaporization, 86 BTU per lb) and then blow the cold vapor over and over the product to remove as much refrigeration as possible before exhausting the vapor from the freezer (a process known as vapor-stripping). Flat belt LN2 tunnels are designed so that the LN2 is sprayed on the food product at the exit end of the freezer, and the cold vapor is forced back toward the entrance of the freezer. In this way, the available refrigeration in the vapor is used most efficiently.
How CO2 Works in a Cryogenic Tunnel Freezer
Liquid CO2 acts very differently in a freezer than liquid nitrogen. CO2 is piped to the tunnel as a high-pressure liquid (300 psi), but once it exits the injection orifice, it instantaneously expands into a mixture of gas and tiny dry ice solid particles (at -109F). The dry ice solid, commonly referred to as dry ice snow, is driven into the surface of the food product, where the heat from the food product rapidly causes the dry ice to sublimate or phase directly from a solid into a gas. The refrigeration effect of CO2 occurs as a result of the latent heat of sublimation (246 BTU per lb of solid CO2, or more commonly represented as 120 BTU per lb of liquid CO2). Where nitrogen tunnels can use refrigeration from both the vaporizing of liquid and warming up the vapor, CO2 tunnels are primarily designed to use refrigeration from dry ice snow sublimation. A CO2 flat belt tunnel looks much the same as a conventional LN2 tunnel, except that LCO2 is injected into the product immediately after it enters the tunnel and almost continuously for about 70% of the length of the tunnel.
How CO2 and LN2 Work in Spiral Freezers
Nitrogen and carbon dioxide are used very similarly in a spiral freezer. Both cryogens are injected across the belt from adjacent manifolds. Both liquid nitrogen and CO2 snow particles are vaporized almost immediately, and extensive air movement from fans located primarily along the outer edges of the belt (vapor stripping) maximizes refrigeration in the vapor form. This freezer type makes highly efficient use of both cryogens.
Misconceptions about these cryogens
We are not an industrial gas supplier; you should consult the industrial gas industry for information related to these products. We urge you to discuss all issues of safety, pricing, product supply, deliveries, and business terms with your industrial gas supplier! If you have decided to utilize a cryogenic freezer, the next decision is to choose which cryogen to use: liquid nitrogen or carbon dioxide.
liquid nitrogen or carbon dioxide major similarities:
* Both of these gases are plentiful throughout most of the country * Both are delivered and stored as a liquid * Both are piped to the freezer… However, major differences include manufacturing, physical properties, and freezer design considerations.
Nitrogen
Approximately 80% of the earth's atmosphere is nitrogen. Nitrogen is separated from air at a separation facility, where air is refrigerated until the major components (nitrogen, oxygen, and argon) are liquefied. Air separation is achieved because these components liquefy at different temperatures.
Carbon Dioxide
Most of the carbon dioxide (CO2) sold to the food processing industry is recovered as a by-product of other chemical processes. The primary sources of CO2 in the United States are ammonia plants, refineries, and ethanol processors, industries that generate a large amount of waste CO2 as a result of manufacturing operations. This waste gas, which would otherwise be vented to the atmosphere, is recovered, cleaned, purified, compressed, and cooled into a liquid. Another significant source of CO2 is natural ground deposits recovered from wells.
Supply Issues
Nitrogen and carbon dioxide will always be available in ample supply because so many essential industries use these products as critical raw materials. However, you should be aware that there are times when regional market shortages occur. Nitrogen shortages can occur when utility companies curtail energy supply to air separation plants (and many other energy-intensive industries). The nitrogen supplier must either reduce their production or be forced to buy power from another source (if available). CO2 shortages occur when by-product source plants (the ammonia plants, refineries, or ethanol processors) cut back production or operations are suspended. This could happen as a result of mechanical difficulties or poor market conditions. Shortages are generally beyond the control of the industrial gas manufacturer. However, the major LN2 and CO2 producers are well aware of their vulnerability to short-term production interruptions and have extensive contingency plans. Most industrial gas companies (both nitrogen and CO2) operate from a network of overlapping plants. If one plant is impacted, a product is usually available from another. However, the industrial gas supplier will incur significant costs in getting through these short-term production interruptions. As part of your gas supplier selection process and to understand how these issues could impact your total freezing cost, we urge you to discuss the following with your potential or existing gas supplier: 1. their supply and distribution capabilities, 2. contingency plans to supply your operations in the event of shortage, 3. how this will impact your cost.
Cryogen Delivery and Storage
Both gases are delivered to you as a refrigerated liquid and shipped in tractors/trailers. A full load of either cryogen is approximately 20 tons. This is a factor that should be considered when you are selecting a storage vessel. If a 20-ton delivery can be made into your storage vessel, then your gas supplier can deliver the product to you at their lowest possible distribution cost, which is a saving that could be beneficial to both parties. Storage vessels for liquid nitrogen are double-walled pressure vessels, normally vertical, with insulation in and a vacuum drawn on the annular space between the vessels. These are called vacuum-jacketed storage vessels, and they are essential to prevent excessive heating of the nitrogen liquid. Nitrogen is usually stored (for freezing applications) at less than 40 psi. Carbon dioxide vessels are also available in a vacuum-jacketed configuration but also as single-walled, insulated pressure vessels (normally horizontal) that are mechanically refrigerated to prevent warming of the liquid CO2. Either configuration is excellent; however, the vacuum-jacketed system requires less maintenance. Liquid CO2 is normally stored at 300 psi. Liquid Nitrogen (LN2) vessels are sized in gallons, and CO2 vessels in tons. This is a rough comparison of standard vessel sizes:
| LN2 Vessel Capacities | CO2 Vessel Capacities | |||||
| Gallons | = | Tons | Tons | = | Gallons | |
| 3,000 | = | 10.2 | 6 | = | 1,408 | |
| 6,000 | = | 20.4 | 14 | = | 3,286 | |
| 9,000 | = | 30.6 | 30 | = | 7,042 | |
| 11,000 | = | 37.4 | ||||
| 13,000 | = | 44.2 | 50 | = | 11,737 | |
Unless your gas supplier recommends otherwise, we would suggest that a 30-ton CO2 vessel, or 9,000 gallons LN2 vessel, be the smallest size you consider. These vessels are usually available for lease from your gas supplier, and some excellent equipment specialists also have lease and service
packages. Storage vessels are usually available for purchase from these same sources. Another difference between the two cryogens is the method in which they are customarily billed. The products you are buying are LIQUID Carbon Dioxide (LCO2) or LIQUID Nitrogen (LN2). LCO2 Liquid carbon dioxide is delivered and billed in pounds. LN2 is often delivered and billed based on its vapor equivalent in 100 standard cubic feet (CCF) units. When
comparing the cost of these cryogens, it is helpful to be able to compare them in terms of cost per pound. Note: what you are really comparing is the cost of BTU, and depending on the type of freezer, The product you are freezing, and the heat transfer efficiency of your system, the BTU value of these cryogens can vary.
| To convert from the cost per CCF of LN2 to the cost per pound of LN2, use this formula:
Price/CCF N2 X .138 = Price/lb LN2 To convert from cost per lb of CO2 to cost per CCF LN2, use this formula: Price/lb CO2 = Price/CCF LN2 _____________ |
Physical Properties of Nitrogen and Carbon Dioxide
| Nitrogen | Carbon Dioxide | |
| Chemical Symbol | N2 | CO2 |
|
|
||
| Latent Heat of Vaporization BTU per LB |
86 | 120 |
|
|
||
| Sensible Heat (gas to 70 °F at one atmosphere) BTU per LB |
98.5 | 29 |
|
|
||
| Total heat to 70 °F (from liquid to 70 °F gas) |
184.5 | 149.1 |
|
|
||
| Latent Heat of Sublimation BTU per LB |
N/A | 246 |
|
|
||
| The Boiling Point of Liquid (at one atmosphere) — °F |
-320 °F | N/A |
|
|
||
| Specific Volume (at standard conditions) — cubic feet/LB |
13.8 | 8.73 |
|
|
||
| LBS liquid per gallon of liquid | 6.8 | 8.52 |
|
|
||
| Standard cubic feet of gas per gallon of liquid | 93.11 | 74.3 |
Purchasing a truckload of liquid nitrogen or liquid CO2 is essentially the same as buying a truckload of BTU. You aim to buy those BTU at the lowest reasonable cost and use them as efficiently as possible in your freezing equipment. Knowing how those BTU are available to you from LN2 or LCO2 is important.
Liquid Nitrogen Refrigeration Looking at the chart above, you will see three BTU values for nitrogen:
Latent Heat of Vaporization, the amount of heat required to vaporize (boil) a pound of liquid nitrogen (86 BTU)
Sensible Heat, the amount of heat to warm nitrogen gas from -320 °F to 70 °F (98.5 BTU)
(gas to a70°F)
Total heat to 70 °F the total of the two values above (184.5 BTU)
Latent Heat of Vaporization, the amount of heat required to vaporize (boil) a pound of liquid nitrogen (86 BTU)
Sensible Heat, the amount of heat to warm nitrogen gas from -320 °F to 70 °F (98.5 BTU)
(gas to a70°F)
Total heat to 70 °F the total of the two values above (184.5 BTU)
What this means to the food processor
When liquid nitrogen is contacted with the product to be frozen, it will immediately deliver up to 86 BTU of refrigeration as it turns from a liquid into a gas. However, the remaining nitrogen gas still contains valuable BTU. CSE designs nitrogen freezers that take advantage of this fact so that we can extract the maximum amount of refrigeration from both liquid and vapor nitrogen.
Liquid Carbon Dioxide Refrigeration
Carbon dioxide can exist as a liquid only at high pressure (such as your storage vessel or pipeline to the freezer). When liquid CO2 is injected into your freezer, the liquid instantly drops to atmospheric pressure. This massive pressure reduction causes the liquid CO2 to phase into a mixture
of solid (dry ice) and gas at a ratio of approximately 45% solid to 55% gas. The gas portion of this mixture has very little refrigeration value, and little attempt has been made in the freezer design to strip the BTU from this vapor, but the solid CO2, commonly known as dry ice snow, has the useable BTU.
When you apply heat to carbon dioxide dry ice, it does not melt. Instead, it sublimates, turning directly from a solid to a gas!
The chart above shows that the Latent Heat of Sublimation for solid CO2 is 246 BTU per pound. Basically, you are buying a pound of liquid CO2 to create .45 pounds of dry ice snow! This dry ice snow is driven into the surface of the food product, where it begins to sublimate and give up its refrigeration. CO2 freezers are designed to maximize the BTU available from dry ice snow.
LCO2 and LN2 are excellent cryogens. Many times, the decision to choose one cryogen over another is purely an economic decision, but there is also a distinct processing advantage based on the cryogen's physical properties, the nature of the product you are freezing, and the type and size of freezer you need.
90 percent of U.S. industrial refrigeration is provided by mechanical systems
More than 90 percent of U.S. industrial refrigeration is provided by mechanical systems using ammonia as the refrigerant. Mechanical refrigeration units are dedicated systems installed at individual industrial facilities and owned and operated by industrial companies. Refrigeration is achieved when the refrigerant, circulating in the system, withdraws heat energy from the chamber to be cooled (load). Heat energy (latent heat of evaporation) is absorbed as the liquid refrigerant changes to a gaseous state. Systems are composed of four basic elements connected with piping into a closed loop that re-circulates refrigerant. Compressors (generally) use motor-driven rotating impellers to generate gas pressure. Gaseous refrigerant enters the compressor at low pressure and temperature and exits at high pressure and temperature. Inside condenser coils, gaseous refrigerant condenses to a liquid state. The condenser dissipates heat energy to facilitate ambient air or water phase change. High-pressure refrigerant exits at lower temperatures. An expansion valve controls the flow of high-pressure liquid refrigerant to the evaporator. As the refrigerant passes through the expansion valve, it is further cooled by the Joule-Thompson effect, the scientific principle that the temperature of a stream is reduced when forced through a narrow nozzle and allowed to expand. Inside the evaporator, liquid refrigerant vaporizes into a gaseous state. Vaporization requires heat energy extracted from the industrial process load (food items to be cooled). The refrigerant is returned to the compressor to repeat the cycle.
High-Pressure Core Heat Exchanger
Heat exchangers are devices that transfer heat from a hot to a cold fluid. The barrier between the two fluids is a metal wall, such as that of a tube or pipe. In many engineering applications, increasing the temperature of one fluid while cooling another is desirable. This double action is economically accomplished by coils, evaporators, condensers, and coolers that may all be considered heat exchangers. Heat exchangers are designed with various flow arrangements. The concentric tube design uses one pipe placed inside another. Cold fluid flows through the inner tube, and warm fluid flows in the same direction through the annular space between the outer and the inner tube. Heat is transferred from the warm fluid through the wall of the inner tube (the so-called heating surface) to the cold fluid. Concentric tube heat exchangers can also be operated in counter-flow, where the two fluids flow in parallel but opposite directions. The shell and tube design utilizes a bundle of tubes through which one of the fluids flows. These tubes are enclosed in a shell with provisions for the other fluid to flow through the spaces between the tubes. In most designs of this type, the free fluid flows roughly perpendicular to the tubes containing the other fluid, in what is known as a cross-flow exchange. The plate-fin design uses metal sheets brazed together into internal channels to carry a warmer fluid stream, which is to be cooled. Fins, brazed to the outside surface of these channels, facilitate faster and more efficient heat transfer to the cold fluid stream on the outside of these channels.
Cryogenic Freezing Systems
Liquid nitrogen or liquid carbon dioxide is purchased and kept in a pressurized storage vessel. The cryogen is piped as a liquid into the freezer unit and applied directly to the product in various modes depending on the cryogen, freezer type, or food product. However, the cryogen is a consumable product and, except in very unusual circumstances, can only be used once. Cryogenic freezing systems are characterized by moderate capital investment, minimal preventive maintenance cost, and a smaller and more flexible commitment to plant space (cryogenic freezers can be installed or removed over a weekend). However, liquid nitrogen or liquid carbon dioxide pricing and availability vary based on the geographic location of the processor, and the cost of refrigeration purchased this way can be more than triple the cost of mechanically produced refrigeration. In contrast to mechanical freezing, cryogenic freezing tends to crust the outside of the product and prevent (insert pictures) excessive moisture loss. Depending on the product, this could be viewed as a quality advantage.
Major Types of Cryogenic Tunnels
Flat Belt Tunnel Freezer
The belt is flat the entire length of the freezer. It can be LN2 or CO2
Multi-pass Tunnel Freezer
The belt is flat the entire length of the freezer, but there are three (or more) stacked belts. At the end of the first tier, the product is discharged to the second tier, returned to the entrance end of the freezer, discharged again onto the third tier, and conveyed back to the exit end. It can be LN2 or CO2
Flighted Belt Tunnel Freezer
The conveyor system is made up of three or more short inclined belts, which tumble the product from one flight to the next. This is excellent for small food products that tend to freeze together when touching. By keeping the individual pieces of food product in motion, large volumes of small food products such as diced meats, berries, or vegetables can be individually quickly frozen (IQF). It can be LN2 or CO2
Immersion Freezer
The food product is conveyed through a bath of liquid nitrogen. It is the fastest heat transfer rate available in cryogenic freezing but is characterized by lower cryogen efficiency rates (higher nitrogen usage) than those achieved with other types of tunnels. However, LN2 immersion freezers mated to spiral freezers (either cryogenic or mechanical) have proven to be highly effective in increasing productivity and yield (reduced dehydration costs) and preventing damage to the bottom of the food product from the spiral belt. LN2 only.
Spiral Freezer
The outward appearance of the spiral is that of a large square box. Inside, the belt spirals around a center drum, each layer a few inches higher. The product is normally fed into the bottom of the spiral and exits at the top of the box. Although heat transfer rates are slower than other cryogenic equipment, this unit can contain as much as 450 feet of belt, allowing for very high production rates. It can be LN2 or CO2
Cabinet (Batch) Freezer
It looks like a large cabinet. Food product is loaded onto trays, slid into a rack, and the rack is pushed into the freezer. The rack and product are removed from the cabinet after freezing is complete. The unit takes up very little floor space, but production is usually limited to a few hundred pounds per hour. Although this is not an inline freezer, its relatively low cost and versatility make this unit a favorite of start-up processors and new product development projects. It can be LN2 or CO2
Tumbler
A long, inclined, rotating tube uses gravity and internal design to convey the product used for small food products that tend to freeze together when touching. Like the flighted freezer, it produces an IQF product. CO2 only
How LN2 Works in a Cryogenic Tunnel
Freezer Most straight belt nitrogen tunnels (the most common type) take advantage of the refrigeration value of converting liquid nitrogen to vapor (Latent Heat of vaporization, 86 BTU per lb.), and then blow the cold vapor over and over the product to remove as much refrigeration as possible before exhausting the vapor from the freezer (a process known as vapor-stripping). Flat belt LN2 tunnels are designed so that the LN2 is sprayed on the food product at the exit end of the freezer, and the cold vapor is forced back toward the entrance of the freezer. In this way, the available refrigeration in the vapor is used most efficiently.
How CO2 Works in a Cryogenic Tunnel Freezer
Liquid CO2 acts very differently in a freezer than liquid nitrogen. CO2 is piped to the tunnel as a high-pressure liquid (300 psi), but once it exits the injection orifice, it instantaneously expands into a mixture of gas and tiny dry ice solid particles (at -109F). The dry ice solid, commonly referred to as dry ice snow, is driven into the surface of the food product, where the heat from the food product rapidly causes the dry ice to sublimate or phase directly from a solid into a gas. The refrigeration effect of CO2 occurs as a result of the latent heat of sublimation (246 BTU per lb of solid CO2, or more commonly represented as 120 BTU per lb of liquid CO2). Where nitrogen tunnels can use refrigeration from both the vaporizing of liquid and warming up the vapor, CO2 tunnels are primarily designed to use refrigeration from dry ice snow sublimation. A CO2 flat belt tunnel freezer looks much the same as a conventional LN2 tunnel, except that LCO2 is injected into the product immediately after it enters the tunnel and almost continuously for about 70% of the length of the tunnel.
How CO2 and LN2 Work in Spiral Freezers
Nitrogen and carbon dioxide are used very similarly in a spiral freezer. Both cryogens are injected across the belt from adjacent manifolds. Both liquid nitrogen and CO2 snow particles are vaporized almost immediately, and extensive air movement from fans located primarily along the outer edges of the belt (vapor stripping) maximizes refrigeration in the vapor form. This freezer type makes highly efficient use of both cryogens.
Misconceptions about these cryogens
We are not an industrial gas supplier; you should consult the industrial gas industry for information on these products. We urge you to discuss all safety issues, pricing, product supply, deliveries, and business terms with your industrial gas supplier! If you have decided to utilize a cryogenic freezer, the next decision is to choose which cryogen to use: liquid nitrogen or carbon dioxide.
Liquid nitrogen or carbon dioxide significant similarities:
* Both gases are plentiful throughout most of the country * Both are delivered and stored as a liquid * Both are piped to the freezer. However, major differences include manufacturing, physical properties, and freezer design considerations.
Nitrogen
Approximately 80% of the earth's atmosphere is nitrogen. Nitrogen is separated from air at a separation facility, where air is refrigerated until the major components (nitrogen, oxygen, and argon) are liquefied. Air separation is achieved because these components liquefy at different temperatures.
Carbon Dioxide
Most of the carbon dioxide (CO2) sold to the food processing industry is recovered as a by-product of other chemical processes. The primary sources of CO2 in the United States are ammonia plants, refineries, and ethanol processors, industries that generate a large amount of waste CO2 as a result of manufacturing operations. This waste gas, which would otherwise be vented to the atmosphere, is recovered, cleaned, purified, compressed, and cooled into a liquid. Another significant source of CO2 is natural ground deposits recovered from wells.
Supply Issues
Nitrogen and carbon dioxide will always be available in ample supply because so many essential industries use these products as critical raw materials. However, you should be aware that there are times when regional market shortages occur. Nitrogen shortages can occur when utility companies curtail energy supply to air separation plants (and many other energy-intensive industries). The nitrogen supplier must either reduce their production or be forced to buy power from another source (if available). CO2 shortages occur when by-product source plants (the ammonia plants, refineries, or ethanol processors) cut back production or operations are suspended. This could happen as a result of mechanical difficulties or poor market conditions. Shortages are generally beyond the control of the industrial gas manufacturer. However, the major LN2 and CO2 producers are well aware of their vulnerability to short-term production interruptions and have extensive contingency plans. Most industrial gas companies (both nitrogen and CO2) operate from a network of overlapping plants. If one plant is impacted, a product is usually available from another. However, the industrial gas supplier will incur significant costs in getting through these short-term production interruptions. As part of your gas supplier selection process and to understand how these issues could impact your total freezing cost, we urge you to discuss the following with your potential or existing gas supplier: 1. their supply and distribution capabilities, 2. contingency plans to supply your operations in the event of shortage, 3. how this will impact your cost.
Cryogen Delivery and Storage
Both gases are delivered to you as a refrigerated liquid and shipped in tractors/trailers. A full load of either cryogen is approximately 20 tons. This is a factor that should be considered when you are selecting a storage vessel. If a 20-ton delivery can be made into your storage vessel, then your gas supplier can deliver the product to you at their lowest possible distribution cost, which is a saving that could be beneficial to both parties. Storage vessels for liquid nitrogen are double-walled pressure vessels, normally vertical, with insulation in and a vacuum drawn on the annular space between the vessels. These are called vacuum-jacketed storage vessels, and they are essential to prevent excessive heating of the nitrogen liquid. Nitrogen is usually stored (for freezing applications) at less than 40 psi. Carbon dioxide vessels are also available in a vacuum-jacketed configuration but also as single-walled, insulated pressure vessels (normally horizontal) that are mechanically refrigerated to prevent warming of the liquid CO2. Either configuration is excellent. however, the vacuum-jacketed system requires less maintenance. Liquid CO2 is normally stored at 300 psi. Liquid Nitrogen (LN2) vessels are sized in gallons, and CO2 vessels in tons. This is a rough comparison of standard vessel sizes:
| LN2 Vessel Capacities | CO2 Vessel Capacities | |||||
| Gallons | = | Tons | Tons | = | Gallons | |
| 3,000 | = | 10.2 | 6 | = | 1,408 | |
| 6,000 | = | 20.4 | 14 | = | 3,286 | |
| 9,000 | = | 30.6 | 30 | = | 7,042 | |
| 11,000 | = | 37.4 | ||||
| 13,000 | = | 44.2 | 50 | = | 11,737 | |
Unless your gas supplier recommends otherwise, we would suggest that a 30-ton CO2 vessel, or 9,000 gallons LN2 vessel, be the smallest size you consider. These vessels are usually available for lease from your gas supplier, and some excellent equipment specialists also have lease and service
packages. Storage vessels are usually available for purchase from these same sources. Another difference between the two cryogens is the method in which they are customarily billed. The products you are buying are LIQUID Carbon Dioxide (LCO2) or LIQUID Nitrogen (LN2). LCO2 Liquid carbon dioxide is delivered and billed in pounds. LN2 is often delivered and billed based on its vapor equivalent in 100 standard cubic feet (CCF) units. When
comparing the cost of these cryogens, it is helpful to be able to compare them in terms of cost per pound. Note: what you are really comparing is the cost of BTU, and depending on the type of freezer, The product you are freezing, and the heat transfer efficiency of your system, the BTU value of these cryogens can vary.
| To convert from the cost per CCF of LN2 to cost per pound of LN2, use this formula:Price/CCF N2 X .138 = Price/lb LN2To convert from cost per lb of CO2 to cost per CCF LN2, use this formula:Price/lb CO2 = Price/CCF LN2 _____________ .138 |
Physical Properties of Nitrogen and Carbon Dioxide
| Nitrogen | Carbon Dioxide | |
| Chemical Symbol | N2 | CO2 |
| Latent Heat of Vaporization BTU per LB |
86 | 120 |
| Sensible heat (gas to 70 °F at one atmosphere) BTU per LB | 98.5 | 29 |
| Total Heat to 70 °F (from liquid to 70 °F gas) | 184.5 | 149.1 |
| Latent Heat of Sublimation BTU per LB | N/A | 246 |
| Boiling Point of Liquid (at one atmosphere) -°F | -320 °F | N/A |
| Specific Volume (at standard conditions) — cubic feet/LB | 13.8 | 8.73 |
| LBS liquid per gallon of liquid | 6.8 | 8.52 |
| Standard cubic feet of gas per gallon of liquid | 93.11 | 74.3 |
Purchasing a truckload of liquid nitrogen or liquid CO2 is essentially the same as buying a truckload of BTU. You aim to buy
those BTU's at the lowest reasonable cost and use them as efficiently as possible in your freezing equipment. Knowing how those BTU's are available to you from LN2 or LCO2 is important. Liquid Nitrogen Refrigeration
Looking at the chart above, you will see three BTU values for nitrogen: Latent Heat of Vaporization, the amount of heat required to vaporize a pound of liquid nitrogen (86 BTU); Sensible Heat (gas to a 70 °F), the amount of heat to warm nitrogen gas from -320 °F to 70 °F (98.5 BTU) Total Heat to 70 °F the total of the two values above (184.5BTUs)Latent Heat of Vaporization the amount of heat required to vaporize (boil) a pound of liquid nitrogen (86 BTU)Sensible Heat (gas to a 70 °F) the amount of heat to warm nitrogen gas from -320 °F to 70 °F (98.5 BTU)Total Heat to 70 °F the total of the two values above (184.5 BTU)What this means to the food processor When liquid nitrogen is put in contact with the product to be frozen, it will deliver up to 86 BTU of refrigeration immediately as it turns from a liquid into a gas. However, there are still valuable BTU in the remaining nitrogen gas. CSE designs nitrogen freezers that take advantage of this fact so that we can extract the maximum amount of refrigeration from both liquid and vapor nitrogen. Liquid Carbon Dioxide, Refrigeration Carbon dioxide can exist as a liquid only at high pressure (such as in your storage vessel or pipeline to the freezer). When liquid CO2 is injected into your freezer, the liquid instantly drops to atmospheric pressure. This massive pressure reduction causes the liquid CO2 to phase into a mixture
of solid (dry ice) and gas at a ratio of approximately 45% solid to 55% gas. The gas portion of this mixture has very little refrigeration value, and little attempt has been made in the freezer design to strip the BTU from this vapor, but the solid CO2, commonly known as dry ice snow, has the useable BTU.NOTE: When you apply heat to carbon dioxide dry ice, it does NOT melt. It sublimates, turning directly from a solid to a gas! The chart above shows that the Latent Heat of Sublimation for solid CO2 is 246 BTU per pound. Basically, you are buying a pound of liquid CO2 to create .45 pounds of dry ice snow! This dry ice snow is driven into the surface of the food product, where it begins to sublimate and give up its refrigeration. CO2 freezers are designed to take maximum advantage of the
BTU is available from dry ice snow.
LCO2 and LN2 are excellent cryogens. There are many times when the decision to choose one cryogen over another is purely an economic decision, but there is also a distinct processing advantage based on the cryogen's physical properties, the nature of the product you are freezing, and the type and size of freezer you need.

